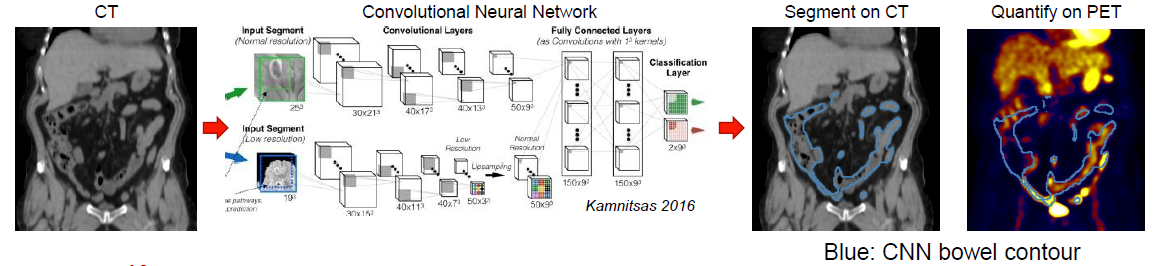
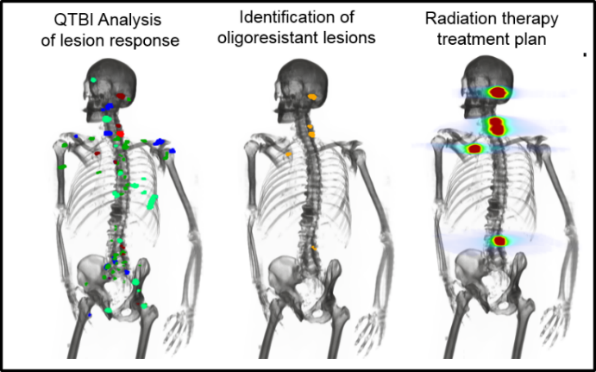
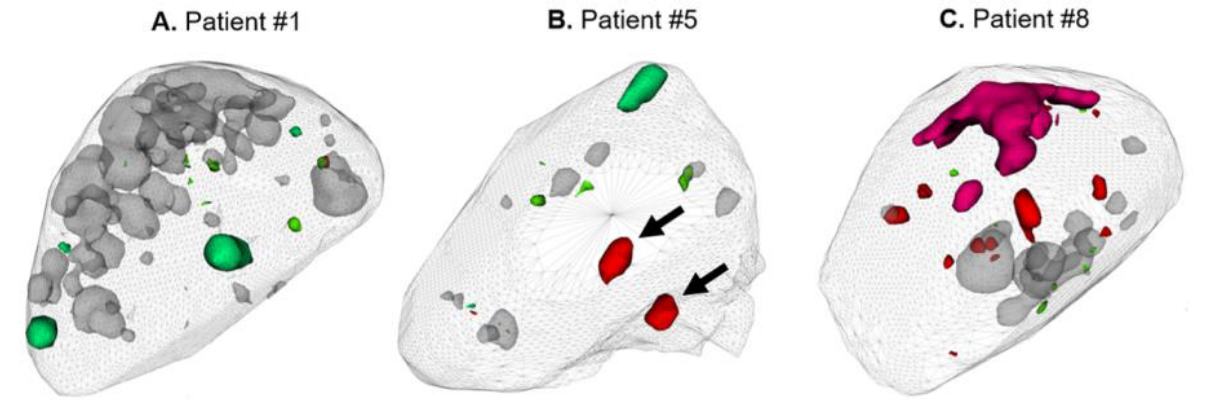
Immune-checkpoint inhibitors (ICI) are effective in the treatment of patients with advanced cancers, such as melanoma; however, patient response rates remain low, and a high prevalence of high-grade immune-related adverse events (irAE) have been reported. Typically, the first response assessment by molecular imaging is made 3-4 months after ICI start; however, small studies have shown that earlier assessment may benefit these patients. This opens an interesting scientific question, whether molecular imaging can provide an earlier, actionable assessment of disease response and irAE development and consequently accelerate clinician decision making to change or maintain treatment.